Atomic Antibiotics Developed by Czech Scientists Counter Bacterial Resistance
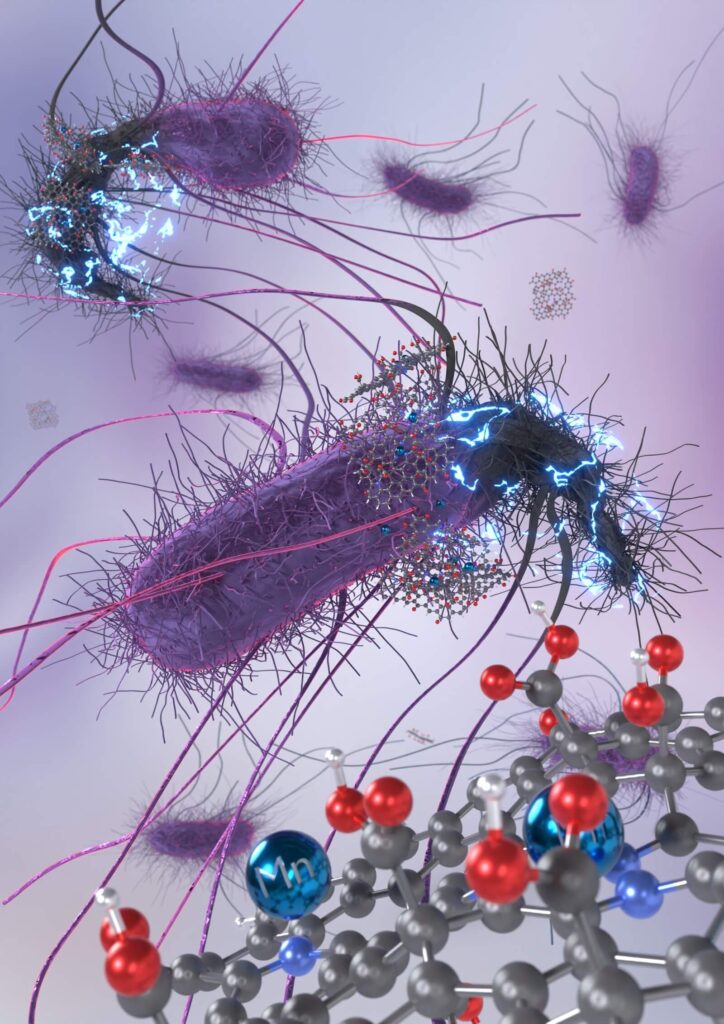
Scientists from Palacký University Olomouc (UP) and the Technical University of Ostrava (VSB-TUO), in collaboration with colleagues from China, have discovered a groundbreaking method for developing a new generation of antibiotics. These antibiotics not only combat a wide range of bacteria but also effectively prevent the development of bacterial resistance. By employing atomic engineering, the researchers transformed manganese—a trace element vital for human health—into a potent antibiotic by embedding it in the structure of chemically modified graphene. Tests conducted on animal models have demonstrated the material’s significant potential, particularly in localized therapies such as wound healing. The discovery has been published in the prestigious journal Advanced Materials, and the team has filed a European patent to safeguard their innovation.
“The material we developed successfully kills and inhibits the growth of all bacteria we studied, including highly resistant pathogens. It operates at low concentrations, which are completely harmless to human cells. Furthermore, bacteria cannot develop resistance to it, thus addressing one of modern medicine’s most pressing challenges. These promising results position atomic antibiotics for practical use in the near future,” said Radek Zbořil, a physical chemist and author of the research concept, who works at the Czech Advanced Technology and Research Institute (CATRIN UP) and the Centre for Energy and Environmental Technologies (CEET) at VSB-TUO.
A Frontal Attack with Manganese
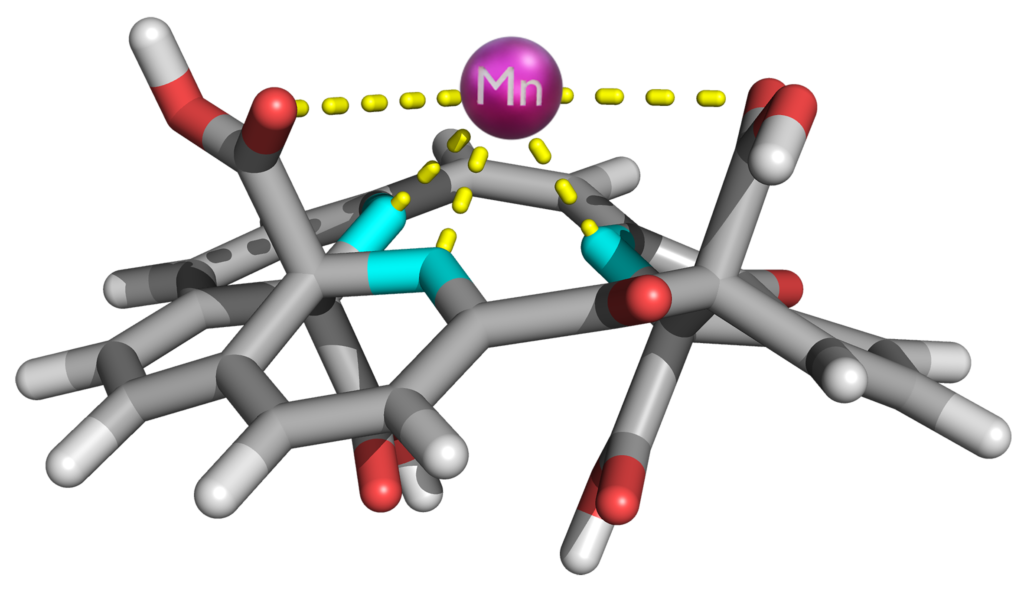
In developing this antibiotic, the scientists drew on their expertise in graphene chemistry and atomic engineering, fields they have extensively studied in recent years. This time, they utilized a graphene derivative enriched with oxygen and nitrogen atoms, chemically incorporating manganese—a transition metal that is involved in metabolism, bone formation, blood sugar regulation and cellular protection against oxidative stress.
“We chose to target one of the strongest defences of bacteria—the carbohydrates in their cell walls and membranes, which are crucial for their survival. These carbohydrates play a protective role, facilitate substance transfer, enable communication with the environment, and serve as energy reserves. By chemically binding manganese to specific carbohydrate groups, we suppressed these critical functions, ultimately causing cell death. The graphene carrier plays an essential role by ensuring the delivery of manganese ions to the bacterial surface, which enables a direct chemical attack on the carbohydrate molecules,” Zbořil explained.
Effective Against Even the Most Resistant Bacteria
The new material also shows remarkable efficacy against bacteria that existing antibiotics struggle to combat. “We observed an excellent bactericidal effect against all bacteria from the ESCAPE group, which includes highly resistant bacterial pathogens. These bacteria are particularly dangerous because they are resistant to conventional antibiotics, which complicates treatment and increases the risk of severe infections, especially in hospital settings. The atomic antibiotic was the only agent that proved effective against all resistant bacteria, compared to commercial antibiotics,” said David Panáček, the first author of the paper, from CATRIN UP and CEET VSB-TUO.
The researchers tested the atomic antibiotic’s effect not only in laboratory settings but also in mouse models in collaboration with their Chinese colleagues. “In in-vivo tests, skin infections caused by resistant strains of Staphylococcus aureus (golden staph) healed quickly and effectively, with all markers of inflammation significantly reduced. We are now considering its use for wound dressings or antibacterial treatments on surfaces of artificial materials. There is tremendous potential to prevent bacterial biofilms from forming on devices such as artificial joint replacements, stents, or cannulas. Given its mode of action, this new material could also help prevent secondary infections, which would have a major impact on healthcare,” said Milan Kolář, a microbiologist and Dean of the Faculty of Medicine and Dentistry at Palacký University Olomouc, who played a key role in the research.
Atomic Antibiotics Open New Possibilities
The research team plans to continue exploring the material’s potential for systemic antibiotic treatments. “Some serious infections are now untreatable with existing antibiotics, and bacterial sepsis is becoming an increasingly common cause of death. We aim to test atomic antibiotics’ effectiveness in treating the most severe bacterial diseases,” Kolář added.
According to the United Nations, if bacterial resistance continues to rise at its current pace, by 2050 untreatable infections caused by multidrug-resistant bacteria could become the leading cause of death worldwide. These so-called ‘superbugs’ pose a global threat, necessitating the development of new antibacterial agents that can bypass the defence mechanisms bacteria use to protect themselves. This research, which also involved colleagues from the Faculty of Science at Palacký University Olomouc and two Chinese institutions, highlights the untapped potential of atomic engineering in addressing one of humanity’s most urgent scientific and social challenges.
The research was supported by the projects TECHSCALE, 2D-BioPAD, and REFRESH.

